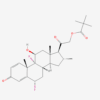
DIFLUCORTOLONE VALERATE
Diflucortolone is a related corticosteroid providing local symptom relief. Side effects include skin irritation and thinning with prolonged use.
About MedicaPharma
MedicaPharma distributes high-quality active pharmaceutical ingredients (APIs) to hospitals, commercial (compounding) pharmacies, research institutes, and universities worldwide.
Network of over +400 GMP API producers
Let us handle your sourcing / supply activities. Highly experienced in sourcing specialty raw pharmaceutical ingredients from niche GMP manufacturers around the world.
Why Choose MedicaPharma
Niche API specialist - Pro-active supply partner - High service level - Global network - Logistics according to GDP regulations
Product Description
Mechanism of Action
Diflucortolone valerate is a potent fluorinated corticosteroid ester designed for high dermal penetration. After enzymatic cleavage of the valerate ester, the active diflucortolone binds cytosolic glucocorticoid receptors (GR), inhibiting transcription of pro‑inflammatory genes, including NF‑κB and AP‑1 pathways. It reduces cytokine synthesis, suppresses leukocyte migration, and stabilizes lysosomal membranes.
Benefits and Advantages
Used in high‑potency topical steroid research, GR‑signalling analysis, cytokine‑modulation studies, dermatologic‑inflammation modelling and corticosteroid ester‑activation assays.
Side Effects and Risks
Risks include marked skin atrophy, telangiectasia, HPA‑axis suppression, contact dermatitis, delayed healing and systemic glucocorticoid effects with prolonged use. Handle with high‑potency steroid precautions.
Datasheet
| Molecular Formula | C27H36F2O6 |
|---|---|
| Molecular Weight | 478.6 g/mol |
| CAS Number | 59198-70-8 |
| Storage Condition | Store in a cool, dry place. Keep container tightly closed. Protect from moisture and light. |
| Solubility | Solubility depends on solvent and conditions (e.g., pH). Please contact us for solvent-specific guidance. |
| Purity | Purity information is available upon request (COA). |
| Synonym | diflucortolone valerate; 59198-70-8; Diflucortolone 21-valerate; Nerisona; DTXSID1048598 |
| IUPAC/Chemical Name | [2-[(6S,8S,9R,10S,11S,13S,14S,16R,17S)-6,9-difluoro-11-hydroxy-10,13,16-trimethyl-3-oxo-7,8,11,12,14,15,16,17-octahydro-6H-cyclopenta[a]phenanthren-17-yl]-2-oxoethyl] pentanoate |
| InChl Key | HHJIUUAMYGBVSD-YTFFSALGSA-N |
| InChl Code | InChI=1S/C27H36F2O5/c1-5-6-7-23(33)34-14-21(31)24-15(2)10-17-18-12-20(28)19-11-16(30)8-9-26(19,4)27(18,29)22(32)13-25(17,24)3/h8-9,11,15,17-18,20,22,24,32H,5-7,10,12-14H2,1-4H3/t15-,17+,18+,20+,22+,24-,25+,26+,27+/m1/s1 |
| References |
3D Conformer.
(Click, turn or enlarge)
Download our GMP API Product List.
MedicaPharma is an EU-based supplier of GMP-certified APIs that serves leading healthcare institutions and research organizations.
Click here to download our full API product list.