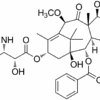
BUSULFAN
Busulfan is an alkylating chemotherapeutic used in chronic myeloid leukemia and transplant conditioning. Side effects include myelosuppression, pulmonary fibrosis, and liver toxicity.
About MedicaPharma
MedicaPharma distributes high-quality active pharmaceutical ingredients (APIs) to hospitals, commercial (compounding) pharmacies, research institutes, and universities worldwide.
Network of over +400 GMP API producers
Let us handle your sourcing / supply activities. Highly experienced in sourcing specialty raw pharmaceutical ingredients from niche GMP manufacturers around the world.
Why Choose MedicaPharma
Niche API specialist - Pro-active supply partner - High service level - Global network - Logistics according to GDP regulations
Product Description
Mechanism of action
Busulfan is a bifunctional alkylating agent that induces DNA interstrand crosslinks through formation of methanesulfonate reactive intermediates. These crosslinks block DNA replication and transcription, triggering cell-cycle arrest, apoptosis and mitotic catastrophe. Busulfan preferentially affects rapidly dividing haematopoietic cells and is widely used in myeloablative conditioning.
Benefits and advantages
Used in oncology research, bone-marrow ablation modelling, DNA-crosslinking mechanism studies, mutagenicity assays, apoptosis pathway research and alkylating-agent SAR profiling. An essential reference compound for studying chemotherapeutic DNA damage.
Side effects and risks
Risks include severe myelosuppression, hepatic veno-occlusive disease, pulmonary fibrosis, seizures (dose-dependent) and gastrointestinal toxicity. Requires full cytotoxic-handling containment.
Datasheet
| Molecular Formula | C6H14O6S2 |
|---|---|
| Molecular Weight | 246.3 g/mol |
| CAS Number | 55-98-1 |
| Storage Condition | Commercially available busulfan for injection concentrate should be stored in unopened ampules at 2-8 °C. The manufacturer states that, when diluted as directed in 0.9% sodium chloride injection or 5% dextrose injection, busulfan solutions are stable for up to 8 hours when stored at room temperature (approximately 25 °C), and the busulfan infusion must be completed during the 8-hour time period. Solutions of busulfan diluted in 0.9% sodium chloride injection also have been shown to be stable when refrigerated at 2-8 °C for up to 12 hours, during which time the infusion must be completed. |
| Solubility | Decomposes (NTP, 1992) |
| Purity | Purity information is available upon request (COA). |
| Synonym | busulfan; 55-98-1; Myleran; Busulphan; Sulphabutin |
| IUPAC/Chemical Name | 4-methylsulfonyloxybutyl methanesulfonate |
| InChl Key | COVZYZSDYWQREU-UHFFFAOYSA-N |
| InChl Code | InChI=1S/C6H14O6S2/c1-13(7,8)11-5-3-4-6-12-14(2,9)10/h3-6H2,1-2H3 |
| References |
3D Conformer.
(Click, turn or enlarge)
Download our GMP API Product List.
MedicaPharma is an EU-based supplier of GMP-certified APIs that serves leading healthcare institutions and research organizations.
Click here to download our full API product list.