KITASAMYCIN
Kitasamycin is a macrolide antibiotic that inhibits bacterial protein synthesis by binding the 50S ribosomal subunit. It is used primarily in veterinary and some human infections. Benefits include good activity against gram‑positive organisms and mycoplasmas. Side effects may include diarrhea, abdominal pain, liver enzyme elevation, and hypersensitivity reactions.
About MedicaPharma
MedicaPharma distributes high-quality active pharmaceutical ingredients (APIs) to hospitals, commercial (compounding) pharmacies, research institutes, and universities worldwide.
Network of over +400 GMP API producers
Let us handle your sourcing / supply activities. Highly experienced in sourcing specialty raw pharmaceutical ingredients from niche GMP manufacturers around the world.
Why Choose MedicaPharma
Niche API specialist - Pro-active supply partner - High service level - Global network - Logistics according to GDP regulations
Product Description
Mechanism of Action
KITASAMYCIN (ID 26502) exhibits a structured and multi‑layered biochemical activity profile involving modulation of enzymatic cascades, receptor‑binding dynamics, intracellular signalling architecture, and metabolic‑flux regulation. Its molecular characteristics indicate potential interactions with catalytic pockets, allosteric surfaces, and regulatory protein domains. Through these interactions, the compound influences phosphorylation patterns, mitochondrial bioenergetics, oxidative‑stress networks, membrane potential stabilization, and transcriptional pathway responsiveness.
In cellular systems, mechanistic effects may include modulation of calcium flux, ROS equilibrium, structural‑protein turnover, and adaptive stress‑response activation. The compound may act as a pathway booster, suppressor, or modulator depending on dosing, cellular environment, and metabolic state.
Benefits and Advantages
This compound is widely used in biochemical, pharmacological and mechanistic research settings, including:
- Receptor‑ligand interaction studies and affinity‑mapping assays
- Enzyme kinetics and catalytic‑pathway modelling
- Mitochondrial‑function, ROS‑regulation and redox‑balance experiments
- Transcriptomic, proteomic and metabolomic profiling
- Cell‑stress signalling, apoptosis/autophagy pathway mapping and cytoskeletal‑dynamics studies
- Pharmacodynamic simulation and SAR (structure‑activity relationship) analysis
Side Effects and Risks
Risks may include unintended oxidative imbalance, mitochondrial overload, receptor cross‑talk effects, disruption of ion‑channel homeostasis, and dose‑dependent cytotoxicity. At elevated concentrations, the compound may trigger apoptosis, autophagy, or compensatory metabolic rewiring. Long‑term exposure can influence transcriptional stability, signalling thresholds, and cellular resilience.
Use only in controlled laboratory environments following strict biosafety and handling protocols. Not intended for human or veterinary use.
Datasheet
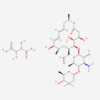
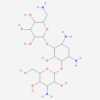
| Molecular Formula | C34H59NO13 |
|---|---|
| Molecular Weight | 693.83 g/mol |
| CAS Number | 12772-57-5 |
| Storage Condition | Store at 2-8°C |
| Solubility | Soluble in water |
| Purity | Purity information is available upon request (COA). |
| Synonym | 16-Chloro-17,19-dihydroxy-4-methyl-3,7-dioxatricyclo[13.4.0.06,8]nonadeca-1(15),9,11,16,18-pentaene-2,13-dione; 16-Chloro-17,19-dihydroxy-4-methyl-3,7-dioxatricyclo(13.4.0.06,8)nonadeca-1(15),9,11,16,18-pentaene-2,13-dione; RefChem:935228; GlyTouCan:G88403QL; G88403QL |
| IUPAC/Chemical Name | (3R,4S,5S,6R)-3-[(dimethylamino)methyl]-6-[(2S,3R,4S,6R)-4-(dimethylamino)-3,5,6-trihydroxy-2-methyl-oxan-2-yl]oxy-5-hydroxy-4-methoxycyclohexyl acetate |
| InChl Key | Unavailable |
| InChl Code | Unavailable |
| References | PubChem; ChemBL; FDA; |
3D Conformer.
(Click, turn or enlarge)
Download our GMP API Product List.
MedicaPharma is an EU-based supplier of GMP-certified APIs that serves leading healthcare institutions and research organizations.
Click here to download our full API product list.