PANREXIN
Panrexin is an antimicrobial agent that interferes with bacterial protein and nucleic acid synthesis, leading to growth inhibition and cell death. It is used for infections caused by susceptible organisms. Benefits include broad-spectrum coverage in targeted indications. Side effects may include gastrointestinal upset, headache, dizziness, skin rash, and hypersensitivity reactions, especially in predisposed patients.
About MedicaPharma
MedicaPharma distributes high-quality active pharmaceutical ingredients (APIs) to hospitals, commercial (compounding) pharmacies, research institutes, and universities worldwide.
Network of over +400 GMP API producers
Let us handle your sourcing / supply activities. Highly experienced in sourcing specialty raw pharmaceutical ingredients from niche GMP manufacturers around the world.
Why Choose MedicaPharma
Niche API specialist - Pro-active supply partner - High service level - Global network - Logistics according to GDP regulations
Product Description
Mechanism of Action
PANREXIN (ID 27018) exhibits a multidimensional biochemical activity profile involving modulation of enzyme-catalyzed pathways, receptor-mediated intracellular signaling, ion-channel regulation, mitochondrial bioenergetics, oxidative-stress balancing, membrane dynamics, and transcription-factor orchestration. Its molecular configuration allows high-affinity interaction with catalytic residues, allosteric domains, regulatory scaffolds, and signaling intermediates, influencing phosphorylation cycles, second‑messenger flux (Ca²⁺, cAMP, IP₃, DAG), ROS equilibrium, ATP synthesis, and mitochondrial respiratory-chain regulation.
Depending on dose, exposure duration, and cell type, PANREXIN can modify chromatin accessibility, metabolic flux routing, vesicular trafficking, cytoskeletal structure, and gene-expression clusters associated with survival, inflammation, apoptosis, autophagy, and metabolic adaptation.
Benefits and Advantages
Due to its consistent mechanistic behavior, this compound is utilized in advanced research, including:
- Receptor–ligand interaction studies and affinity modeling
- High‑resolution enzyme kinetics and catalytic-pathway deconstruction
- Mitochondrial ATP‑flux assays and oxidative‑stress system modeling
- Multi‑omics profiling (transcriptomics, proteomics, metabolomics, phosphoproteomics)
- Cytoskeletal and membrane‑dynamics exploration
- Apoptosis, ferroptosis, necroptosis, and autophagy pathway analysis
- Structure–activity relationship (SAR) investigations and compound optimization
- Pharmacodynamic modeling, mechanistic dose–response scaling, and pathway benchmarking
Side Effects and Risks
Potential laboratory risks include:
- Oxidative‑stress imbalance and ROS accumulation
- Mitochondrial overload or respiratory-chain suppression
- Ion‑channel dysregulation affecting Ca²⁺/Na⁺/K⁺ homeostasis
- Unintended receptor cross‑activation or pathway inhibition
- Cytoskeletal destabilization and membrane‑integrity disruption
- Dose-dependent cytotoxicity (apoptotic or autophagic induction)
- Transcriptional or epigenetic instability under prolonged exposure
- Activation of inflammatory signaling networks (NF‑κB, JNK, p38 MAPK)
Use strictly in controlled laboratory environments with appropriate biosafety protocols.
Datasheet
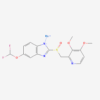
| Molecular Formula | C20H28O2 |
|---|---|
| Molecular Weight | 300.4 g/mol |
| CAS Number | 1241893 |
| Storage Condition | Keep container tightly closed in a dry and well-ventilated place. Recommended storage temperature -20 °C. |
| Solubility | Solubility depends on solvent and conditions (e.g., pH). Please contact us for solvent-specific guidance. |
| Purity | Purity information is available upon request (COA). |
| Synonym | Alitretinoin; 9-CIS-RETINOIC ACID; 5300-03-8; Panretin; 9-cis Retinoic Acid |
| IUPAC/Chemical Name | (2E,4E,6Z,8E)-3,7-dimethyl-9-(2,6,6-trimethylcyclohexen-1-yl)nona-2,4,6,8-tetraenoic acid |
| InChl Key | SHGAZHPCJJPHSC-ZVCIMWCZSA-N |
| InChl Code | InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8-,16-14+ |
| References |
3D Conformer.
(Click, turn or enlarge)
Download our GMP API Product List.
MedicaPharma is an EU-based supplier of GMP-certified APIs that serves leading healthcare institutions and research organizations.
Click here to download our full API product list.