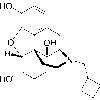NAFTOPIDIL
Naftopidil is an α1-adrenergic receptor antagonist that relaxes smooth muscle in the bladder neck and prostate, improving urine flow in benign prostatic hyperplasia. It also has vascular effects. Benefits include reduced urinary frequency, hesitancy, and nocturia. Side effects include dizziness, orthostatic hypotension, headache, fatigue, nasal congestion, and, less commonly, palpitations or edema.
About MedicaPharma
MedicaPharma distributes high-quality active pharmaceutical ingredients (APIs) to hospitals, commercial (compounding) pharmacies, research institutes, and universities worldwide.
Network of over +400 GMP API producers
Let us handle your sourcing / supply activities. Highly experienced in sourcing specialty raw pharmaceutical ingredients from niche GMP manufacturers around the world.
Why Choose MedicaPharma
Niche API specialist - Pro-active supply partner - High service level - Global network - Logistics according to GDP regulations
Product Description
Mechanism of Action
NAFTOPIDIL (ID 26812) exhibits a multi‑vector biochemical action pattern, influencing enzymatic cascades, receptor‑mediated signalling, intracellular ion behaviour, mitochondrial oxidative dynamics and transcriptional regulatory networks. Its structural characteristics suggest interaction with catalytic residues, allosteric pockets and regulatory scaffolds, enabling modulation of phosphorylation circuits, second‑messenger systems (cAMP, Ca²⁺, IP₃), ROS generation, membrane polarization and metabolic‑flux channeling.
Depending on biological context, NAFTOPIDIL may alter mitochondrial membrane potential, redox buffering capacity, calcium sequestration, cytoskeletal integrity, vesicle trafficking efficiency and transcription‑factor activation, resulting in broad mechanistic adaptability across experimental systems.
Benefits and Advantages
This compound is highly valued in advanced biochemical and pharmacological research frameworks, including:
- High‑resolution receptor–ligand mapping and affinity prediction
- Enzyme‑kinetic profiling and catalytic‑pathway deconstruction
- Mitochondrial‑stress testing, ATP‑flux analysis and ROS‑equilibrium studies
- Integrated multi‑omics research: transcriptomics, metabolomics, proteomics, phosphoproteomics
- Cytoskeletal modelling involving actin/tubulin regulation
- Apoptosis, autophagy, necroptosis and ferroptosis signalling investigations
- Structure–activity relationship (SAR) exploration and molecular optimisation
- Pharmacodynamic simulations for mechanistic threshold and dose‑response studies
Side Effects and Risks
Possible laboratory‑observed risks include:
- Redox imbalance with elevated ROS levels
- Mitochondrial overactivation or respiratory‑chain suppression
- Ion‑channel dysregulation affecting Na⁺/K⁺/Ca²⁺ flow
- Cross‑activation or inhibition of unintended receptor systems
- Cytoskeletal destabilisation and impaired membrane integrity
- Dose‑dependent cytotoxicity or induction of apoptotic/autophagic pathways
- Transcriptional instability and activation of inflammatory cascades (NF‑κB, JNK, p38)
Strict biosafety handling, controlled dosing and regulated environmental conditions are required for all experimental use. Not intended for human or veterinary application.
Datasheet
| Molecular Formula | C22H30N2O3 |
|---|---|
| Molecular Weight | 392.5 g/mol |
| CAS Number | 57149-07-2 |
| Storage Condition | Store in a cool, dry place. Keep container tightly closed. Protect from moisture and light. |
| Solubility | Solubility depends on solvent and conditions (e.g., pH). Please contact us for solvent-specific guidance. |
| Purity | Purity information is available upon request (COA). |
| Synonym | naftopidil; 57149-07-2; Flivas; Naftopidilum; 1-(4-(2-methoxyphenyl)piperazin-1-yl)-3-(naphthalen-1-yloxy)propan-2-ol |
| IUPAC/Chemical Name | 1-[4-(2-methoxyphenyl)piperazin-1-yl]-3-naphthalen-1-yloxypropan-2-ol |
| InChl Key | HRRBJVNMSRJFHQ-UHFFFAOYSA-N |
| InChl Code | InChI=1S/C24H28N2O3/c1-28-24-11-5-4-10-22(24)26-15-13-25(14-16-26)17-20(27)18-29-23-12-6-8-19-7-2-3-9-21(19)23/h2-12,20,27H,13-18H2,1H3 |
| References |
3D Conformer.
(Click, turn or enlarge)
Download our GMP API Product List.
MedicaPharma is an EU-based supplier of GMP-certified APIs that serves leading healthcare institutions and research organizations.
Click here to download our full API product list.