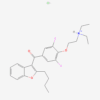
AMINOPYRIN
Aminopyrin is an antipyretic and analgesic that inhibits prostaglandin formation. Due to toxicity risks, its use is limited. Side effects include agranulocytosis and hypersensitivity.
About MedicaPharma
MedicaPharma distributes high-quality active pharmaceutical ingredients (APIs) to hospitals, commercial (compounding) pharmacies, research institutes, and universities worldwide.
Network of over +400 GMP API producers
Let us handle your sourcing / supply activities. Highly experienced in sourcing specialty raw pharmaceutical ingredients from niche GMP manufacturers around the world.
Why Choose MedicaPharma
Niche API specialist - Pro-active supply partner - High service level - Global network - Logistics according to GDP regulations
Product Description
Mechanism of action
Aminopyrin inhibits COX enzymes to reduce prostaglandin production, mediating analgesic and antipyretic effects. More importantly, its metabolism to reactive intermediates such as 4‑aminoantipyrine accounts for its historical toxicity and relevance in pharmacokinetic probe studies.
Benefits and advantages
Still used in the aminopyrine breath test to measure hepatic demethylation capacity and microsomal oxidative function. Highly valuable in hepatology research.
Side effects and risks
Agranulocytosis, hepatic necrosis, hypersensitivity, shock-like reactions. Requires strict PPE and dedicated ventilation.
Datasheet
| Molecular Formula | C13H17N3O |
|---|---|
| Molecular Weight | 231.29 g/mol |
| CAS Number | 58-15-1 |
| Storage Condition | …MATERIALS WHICH…CAN DECOMP INTO TOXIC COMPONENTS DUE TO CONTACT WITH HEAT…STORED IN COOL, WELL-VENTILATED PLACE, OUT OF…RAYS OF SUN, AWAY FROM AREAS OF HIGH FIRE HAZARD…PERIODICALLY INSPECTED & MONITORED. INCOMPATIBLE MATERIALS…ISOLATED FROM EACH OTHER. |
| Solubility | greater than or equal to 100 mg/mL at 72 °F (NTP, 1992) |
| Purity | Purity information is available upon request (COA). |
| Synonym | aminophenazone; AMINOPYRINE; Amidopyrine; 58-15-1; 4-Dimethylaminoantipyrine |
| IUPAC/Chemical Name | 4-(dimethylamino)-1,5-dimethyl-2-phenylpyrazol-3-one |
| InChl Key | RMMXTBMQSGEXHJ-UHFFFAOYSA-N |
| InChl Code | InChI=1S/C13H17N3O/c1-10-12(14(2)3)13(17)16(15(10)4)11-8-6-5-7-9-11/h5-9H,1-4H3 |
| References |
3D Conformer.
(Click, turn or enlarge)
Download our GMP API Product List.
MedicaPharma is an EU-based supplier of GMP-certified APIs that serves leading healthcare institutions and research organizations.
Click here to download our full API product list.