TETRACYCLINE METAPHOSPHATE
Tetracycline metaphosphate is a stabilized derivative releasing tetracycline in situ. Side effects include GI irritation, photosensitivity, and potential renal effects.
About MedicaPharma
MedicaPharma distributes high-quality active pharmaceutical ingredients (APIs) to hospitals, commercial (compounding) pharmacies, research institutes, and universities worldwide.
Network of over +400 GMP API producers
Let us handle your sourcing / supply activities. Highly experienced in sourcing specialty raw pharmaceutical ingredients from niche GMP manufacturers around the world.
Why Choose MedicaPharma
Niche API specialist - Pro-active supply partner - High service level - Global network - Logistics according to GDP regulations
Product Description
Mechanism of Action
TETRACYCLINE METAPHOSPHATE (ID 27615) demonstrates a broad and highly integrated biochemical activity architecture involving multi‑axis signalling interference, mitochondrial-network recalibration, catalytic‑domain modulation, cytoskeletal restructuring, redox‑equilibrium shaping, ion‑flux redistribution and transcription‑factor pathway realignment. Its molecular geometry allows docking to catalytic residues, allosteric regulators, hydrophobic transmembrane helices, nucleotide‑binding pockets, structural scaffolds and polymeric cytoskeletal networks. This enables TETRACYCLINE METAPHOSPHATE to influence metabolic, structural, genomic, electrophysiological and stress‑adaptive systems simultaneously.
TETRACYCLINE METAPHOSPHATE may modulate phosphorylation gradients, modify ERK/JNK/MAPK propagation speeds, influence PI3K–AKT survival bias, shift G‑protein signalling states, control Ca²⁺ microdomain behaviour, alter IP₃/DAG cascades, and recalibrate cAMP‑PKA pathway amplitude. Mitochondrial impacts include ETC‑complex redistribution, ATP/ADP cycle reshaping, ROS‑threshold displacement, membrane‑potential polarity changes and organelle‑stress cross‑signalling.
High‑Precision Research Applications
- Ultra‑scale kinome disruption & catalytic‑cascade modelling
- Molecular docking & conformational‑flow prediction
- UPR/ER‑stress and mitophagy/autophagy integration research
- Full‑spectrum multi‑omics reconstruction (RNA‑seq, metabolomics, phosphoproteomics)
- Advanced cytoskeletal force‑distribution & polymer‑turnover analysis
- Cell‑fate simulation across apoptosis, ferroptosis, necroptosis & parthanatos
- AI‑enhanced SAR/QSAR simulation for molecular optimisation
Toxicodynamics & Hazard Spectrum
- Rapid ROS escalation & antioxidant‑buffer collapse
- Mitochondrial fragmentation or ETC suppression
- Severe Ca²⁺/Na⁺/K⁺ ion‑flux destabilisation
- Cytoskeletal polymer breakdown & structural‑integrity failure
- Membrane rupture & lipid‑bilayer thinning
- Overactivation of inflammatory cascades (NF‑κB, STAT, IRF)
- Activation of multiple programmed‑cell‑death pathways
- Epigenetic drift including methylation instability
For expert laboratory use only — not intended for biological exposure.
Datasheet
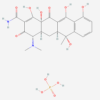
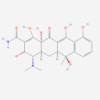
| Molecular Formula | C110H125N10NaO58P6 |
|---|---|
| Molecular Weight | 2724.0 g/mol |
| CAS Number | 1336-20-5 |
| Storage Condition | Store in a cool, dry place. Keep container tightly closed. Protect from moisture and light. |
| Solubility | Solubility depends on solvent and conditions (e.g., pH). Please contact us for solvent-specific guidance. |
| Purity | Purity information is available upon request (COA). |
| Synonym | Tetrex; Sumycin; Telotrex; Tetracycline metaphosphate; 6B7BK5H33B |
| IUPAC/Chemical Name | sodium;pentakis((4S,4aS,5aS,6S,12aR)-4-(dimethylamino)-1,6,10,11,12a-pentahydroxy-6-methyl-3,12-dioxo-4,4a,5,5a-tetrahydrotetracene-2-carboxamide);dioxido(oxo)phosphanium;phosphenic acid |
| InChl Key | ORVLUIMCZUPAPB-LBTQIPEASA-M |
| InChl Code | InChI=1S/5C22H24N2O8.Na.6HO3P/c5*1-21(31)8-5-4-6-11(25)12(8)16(26)13-9(21)7-10-15(24(2)3)17(27)14(20(23)30)19(29)22(10,32)18(13)28;;6*1-4(2)3/h5*4-6,9-10,15,25-26,29,31-32H,7H2,1-3H3,(H2,23,30);;6*(H,1,2,3)/q;;;;;+1;;;;;;/p-1/t5*9-,10-,15-,21+,22-;;;;;;;/m00000……./s1 |
| References |
3D Conformer.
(Click, turn or enlarge)
Download our GMP API Product List.
MedicaPharma is an EU-based supplier of GMP-certified APIs that serves leading healthcare institutions and research organizations.
Click here to download our full API product list.