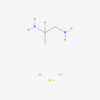
ALOSETRON
Alosetron is a selective 5‑HT3 receptor antagonist used for severe irritable bowel syndrome with diarrhea in women. Benefits include reduced urgency and abdominal discomfort. Risks include constipation, ischemic colitis, and serious gastrointestinal complications, requiring restricted use.
About MedicaPharma
MedicaPharma distributes high-quality active pharmaceutical ingredients (APIs) to hospitals, commercial (compounding) pharmacies, research institutes, and universities worldwide.
Network of over +400 GMP API producers
Let us handle your sourcing / supply activities. Highly experienced in sourcing specialty raw pharmaceutical ingredients from niche GMP manufacturers around the world.
Why Choose MedicaPharma
Niche API specialist - Pro-active supply partner - High service level - Global network - Logistics according to GDP regulations
Product Description
Mechanism of action
Alosetron is a highly selective antagonist of the serotonin 5-HT3 receptor, which is expressed on enteric neurons and visceral afferent fibres. By blocking these receptors in the gastrointestinal tract, alosetron reduces afferent signalling from the gut to the central nervous system, leading to decreased visceral pain perception, slower colonic transit and reduced intestinal secretion. This combination of effects helps normalise bowel frequency and stool consistency in patients with diarrhoea-predominant irritable bowel syndrome (IBS-D).
Benefits and advantages
The compound provides targeted symptom relief in carefully selected patients with severe, chronic IBS-D who have not responded adequately to conventional therapies. Clinical use has shown meaningful improvements in stool urgency, frequency, consistency and abdominal discomfort, which can significantly enhance quality of life. Because of its receptor selectivity, alosetron has minimal sedative or systemic neurological effects compared with less specific serotonergic agents.
Side effects and risks
The major safety concern with alosetron is the risk of serious gastrointestinal adverse events, including severe constipation, ischaemic colitis and, rarely, intestinal perforation. For this reason its therapeutic use is restricted to specific patient populations under strict prescribing programmes in clinical practice. Other adverse effects include abdominal pain, nausea and headache. As an active pharmaceutical ingredient, alosetron must be handled only by trained professionals under appropriate containment and safety procedures.
Datasheet
| Molecular Formula | C17H18N4O |
|---|---|
| Molecular Weight | 294.35 g/mol |
| CAS Number | 122852-42-0 |
| Storage Condition | Store in a cool, dry place. Keep container tightly closed. Protect from moisture and light. |
| Solubility | 4.38e-01 g/L |
| Purity | Purity information is available upon request (COA). |
| Synonym | alosetron; 122852-42-0; GR68755; alosetron monohydrochloride; 5-methyl-2-[(5-methyl-1H-imidazol-4-yl)methyl]-3,4-dihydropyrido[4,3-b]indol-1-one |
| IUPAC/Chemical Name | 5-methyl-2-[(5-methyl-1H-imidazol-4-yl)methyl]-3,4-dihydropyrido[4,3-b]indol-1-one |
| InChl Key | JSWZEAMFRNKZNL-UHFFFAOYSA-N |
| InChl Code | InChI=1S/C17H18N4O/c1-11-13(19-10-18-11)9-21-8-7-15-16(17(21)22)12-5-3-4-6-14(12)20(15)2/h3-6,10H,7-9H2,1-2H3,(H,18,19) |
| References |
3D Conformer.
(Click, turn or enlarge)
Download our GMP API Product List.
MedicaPharma is an EU-based supplier of GMP-certified APIs that serves leading healthcare institutions and research organizations.
Click here to download our full API product list.